- Isolation and Identification of Amino Acids, Vine and Carboxylic
- Isolation and Identification of Amino Acids, Vine and Carboxylic
- ازمایش 3 Making alcohol-derived data
- Alcohols
- alchool test
- Aldehyde & Ketons
- Isolation and Identification of Amino Acids, Vine and Carboxylic
- Identify alkene and alkyne, halide of aliphatic, Identifyand carbohydrates
- Affinity chromatography
آخرین مطالب
امکانات وب
 Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively.
Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest.
Amine function Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen.
Amines Application:
Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids. In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are various uses in the industry. Methamidamine (HMDA) is a commercially important amine. Which is the nylon industrial nylon material.
Solubility test A compound that is insoluble in water and not in solution, but dissolved in 5% hydrochloric acid soluti Affinity chromatography...
Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively.
Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest.
Amine function Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen.
Amines Application:
Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids. In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are various uses in the industry. Methamidamine (HMDA) is a commercially important amine. Which is the nylon industrial nylon material.
Solubility test A compound that is insoluble in water and not in solution, but dissolved in 5% hydrochloric acid soluti Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 94
 Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively.
Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest.
Amine function
Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen.
Amines Application:
Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids.
In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are various us Affinity chromatography...
Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively.
Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest.
Amine function
Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen.
Amines Application:
Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids.
In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are various us Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 229
. Alcohol, the most common derivative derivative. making spirits 3 and 5-Nitro phenyl benzoate, ethyl acetate and aortanha. You may also use the alpha-نفتیل polyurethane but the test used for more fnlaha. In all cases after the product was pure solid Crystal melting point eas using the reference tables, the type of alcohol. Preparation of 3 and 5 di Nitro benzoate: combine 3 and 5 Dec نیتروبنزوئیل chloride with alcohols, Ester comes and get the corresponding procedures for the first, second and third type of alcohols. Especially for الکلهایی that dissolve in water and may have a very slight amount of water can be helpful
1) liquid alcohols:
in a test tube is completely dry 2 30 30 hot with alcohol about half 3و5 di Nitro بنزوییل chloride mixture, and continue to simmer gently for 5 minutes. About 10 30 30 ml distilled water and add it to the solution in an ice bath to cool the product into the hands of China. Collect the raw product and with 10 30 30 2% sodium carbonate and water-solution using the atanolmajdda field. Solvent for recrystallization to finalise the work must be a minimum size to be
۲)of solid alchol :
solid alcohol at 5 30 30 and a half grams of dry-diaminopyridin Affinity chromatography...ادامه مطلب
ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 240
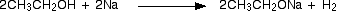 Alcohols
Alcohols are classified into three types of type I, type II or type III, depending on the type of carbon bonded to the hydroxyl group (OH)
The carbon atom of the first and second types of alcohol, in contrast to the third type of alcohol, is subject to oxidation. In order to oxidize the alcohols, it is necessary to have a carbon atom attached to the hydroxyl agent, at least one hydrogen atom. From the oxidation of the first type of alcohol, first, aldehydes are produced, and then the acid is produced and, by oxidation, second-order alcohols only become ketones. Third-grade alcohols do not participate in oxidative reactions
On the other hand, type III alcohols produce more stable ions (carboxytones) with the loss of the OH functional group. Carboxyhounds are positive ions that alkylated electron substitutions, by stabilizing the positive charge, increase their stability and longevity. Thus, type 3 alcohols produce more stable carboxyates
Physical Properties of Alcohols
The general formula for alcohols is R_OH in which R is an alkyl or alkyl group. The general formula is a non-ring CnH2n + 1 simple alcohol.It may be an open or circular chain; it may have a halogen atom Affinity chromatography...
Alcohols
Alcohols are classified into three types of type I, type II or type III, depending on the type of carbon bonded to the hydroxyl group (OH)
The carbon atom of the first and second types of alcohol, in contrast to the third type of alcohol, is subject to oxidation. In order to oxidize the alcohols, it is necessary to have a carbon atom attached to the hydroxyl agent, at least one hydrogen atom. From the oxidation of the first type of alcohol, first, aldehydes are produced, and then the acid is produced and, by oxidation, second-order alcohols only become ketones. Third-grade alcohols do not participate in oxidative reactions
On the other hand, type III alcohols produce more stable ions (carboxytones) with the loss of the OH functional group. Carboxyhounds are positive ions that alkylated electron substitutions, by stabilizing the positive charge, increase their stability and longevity. Thus, type 3 alcohols produce more stable carboxyates
Physical Properties of Alcohols
The general formula for alcohols is R_OH in which R is an alkyl or alkyl group. The general formula is a non-ring CnH2n + 1 simple alcohol.It may be an open or circular chain; it may have a halogen atom Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 179
 Effective factors in solubilityPolar compounds are usually dissolved in polar solvents and non-polar compounds in non-polar solvents.In similar compositions, increasing intermolecular force reduces solubility.In similar compositions, molecular weight gain reduces solubilityIn the same combinations, the lateral branch increases solubilit
. Use of solubility in identifying an unknown objectThe use of solubility to some extent determines the chemical properties of an organic body. For example, acidic compounds usually dissolve in 5% chloride acid in alkaline and alkaline compounds. Uses information solvability for some of the unknown combination properties. By solubilizing an object in water, we are somewhat sure about its polarity, or while benzoic acid is not dissolved in water, but if combined with profit, it produces sodium benzoate, which is readily soluble in water. The use of solubility gives information about the molecular weight of an unknown object, for example, in the case of homologous series having a chemical agent, those whose carbon number is less than 4 in solution, and those whose carbon number is greater than 5 atoms Carbon is usually insoluble in water.
Solvency Affinity chromatography...
Effective factors in solubilityPolar compounds are usually dissolved in polar solvents and non-polar compounds in non-polar solvents.In similar compositions, increasing intermolecular force reduces solubility.In similar compositions, molecular weight gain reduces solubilityIn the same combinations, the lateral branch increases solubilit
. Use of solubility in identifying an unknown objectThe use of solubility to some extent determines the chemical properties of an organic body. For example, acidic compounds usually dissolve in 5% chloride acid in alkaline and alkaline compounds. Uses information solvability for some of the unknown combination properties. By solubilizing an object in water, we are somewhat sure about its polarity, or while benzoic acid is not dissolved in water, but if combined with profit, it produces sodium benzoate, which is readily soluble in water. The use of solubility gives information about the molecular weight of an unknown object, for example, in the case of homologous series having a chemical agent, those whose carbon number is less than 4 in solution, and those whose carbon number is greater than 5 atoms Carbon is usually insoluble in water.
Solvency Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 198
 Aldehyde & Ketones
In compounds having a carbonyl functional group, if the carbonyl group is substituted with hydrogen atoms or alkylated groups, they are called aldehyde (RCHO) or ketone (RCOR). The chemistry of these compounds, in fact, is the chemical group of carbonyl derivatives. Identification of these compounds is possible by the characteristic reactions of the carbonyl functional group. To identify aldehydes and ketones there are various tests that can be used to test 2 and 4 - di nitrofenylhydrazine, chromic acid test, iodoform test, talenz test, fushin test and benedic test.
2,4dinitrofenylhydrazine
This test is the most prominent test for the detection of aldehydes and ketones. Aldehydes and ketones with a reagent 2 and 4-di-nitro-phenyl hydrazine form a yellow to orange deposit (red).Aldehyde and ketones that have a carbonaceous conjugate and are unsaturated are orange (red), i.e. with higher wavelengths
In this nuclear reaction, nitrogen friendliness makes the reaction easy to accomplish
This test, in addition to identifying aldehyde or ketone, is also one of the derivatives of these compounds, since the formed sediment is polarized, flattened and heavie Affinity chromatography...
Aldehyde & Ketones
In compounds having a carbonyl functional group, if the carbonyl group is substituted with hydrogen atoms or alkylated groups, they are called aldehyde (RCHO) or ketone (RCOR). The chemistry of these compounds, in fact, is the chemical group of carbonyl derivatives. Identification of these compounds is possible by the characteristic reactions of the carbonyl functional group. To identify aldehydes and ketones there are various tests that can be used to test 2 and 4 - di nitrofenylhydrazine, chromic acid test, iodoform test, talenz test, fushin test and benedic test.
2,4dinitrofenylhydrazine
This test is the most prominent test for the detection of aldehydes and ketones. Aldehydes and ketones with a reagent 2 and 4-di-nitro-phenyl hydrazine form a yellow to orange deposit (red).Aldehyde and ketones that have a carbonaceous conjugate and are unsaturated are orange (red), i.e. with higher wavelengths
In this nuclear reaction, nitrogen friendliness makes the reaction easy to accomplish
This test, in addition to identifying aldehyde or ketone, is also one of the derivatives of these compounds, since the formed sediment is polarized, flattened and heavie Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 201
 Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively. Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest. Amine function Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen. Amines Application: Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids. In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are va Affinity chromatography...
Amines are organic compounds and are considered to be components of the water (the color of the litmus lamp is blue), which includes a central nitrogen atom and one, two or three groups of alkyl with general formulas NR 3 _NHR 2 _NH 2 R They are called amine type 1, amine type 2, and amine type 3, respectively. Amines are usually extracted from ammonia, which is replaced by alkylated hydrogens. The second type of amine play is more than the rest. Amine function Amines and other nitrogen-containing compounds are among the most abundant organic molecules. All amines have the character of the game (first and second type amines can act as acids), they form hydrogen bonds and act as a keel in substitution reactions. So in many respects, chemistry is similar to that of alcohols and ethers. But there are differences in activity as electronegative nitrogen is less than oxygen. Amines Application: Many biological active compounds contain nitrogen. Many simple amines are used as drugs. Like morphine and codeine in the amine group. Other important amines are amino acids. In addition to the use of amines in pharmaceuticals and the separation of enantiomers, there are va Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 249
 Specific identification tests are not detected by chemical means. Undertakings do not show in their inadequate and unconventional environment their physical attributes for identification.The alkene group is a carbon-carbon double carbon bond. Therefore, in order to identify an unknown alkanical compound, it should be shown that the ordinary reactions of the carbon-carbon double bond are performed. Due to the large number of such reactions, this may seem easy.If possible, we select a reaction as an identification test that can be done quickly and easily and can lead to a visible change. We will select a test, which will require a few minutes and several test pipes, a test in which the color will be created or destroyed, or the bubbles will come out, or the sediment will be formed or dissolved
Chemical properties
Bromine will add to the carbon-carbon double bond of alkenes to produce dibromoalkanes and with alkynes to produce tetrabromoalkanes. When this reaction occurs, molecular bromine is consumed, and its characteristic dark red‑brown color disappears if bromine is not added in excess. The rapid disappearance of the bromine color is a positive test for unsaturation
The test is Affinity chromatography...
Specific identification tests are not detected by chemical means. Undertakings do not show in their inadequate and unconventional environment their physical attributes for identification.The alkene group is a carbon-carbon double carbon bond. Therefore, in order to identify an unknown alkanical compound, it should be shown that the ordinary reactions of the carbon-carbon double bond are performed. Due to the large number of such reactions, this may seem easy.If possible, we select a reaction as an identification test that can be done quickly and easily and can lead to a visible change. We will select a test, which will require a few minutes and several test pipes, a test in which the color will be created or destroyed, or the bubbles will come out, or the sediment will be formed or dissolved
Chemical properties
Bromine will add to the carbon-carbon double bond of alkenes to produce dibromoalkanes and with alkynes to produce tetrabromoalkanes. When this reaction occurs, molecular bromine is consumed, and its characteristic dark red‑brown color disappears if bromine is not added in excess. The rapid disappearance of the bromine color is a positive test for unsaturation
The test is Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 250
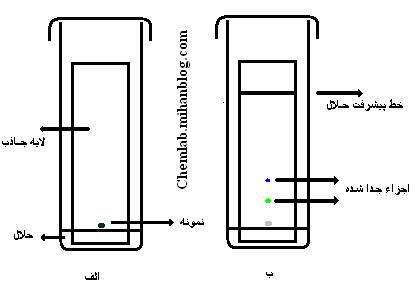 Thin Film Chromatography (TLC): Thin-layer chromatography is a type of , in which thin-film plates are used and the position of the components on the plate is determined. The particles on the layer should be large and compact and small. The quateary is often made up of , which is used to separate hydrophilic molecules such as carbon hydrates , amino acids , and Initially, chromatographic plates are required, ie, the gravity is distributed as a thin layer of uniform thickness on an inert rigid support. Glass sheets are commonly used, but there are other methods. Solid adsorbent in the form of fine powder with water, and sometimes with an escaping liquid , in the form of dough, and distributed by a commercial player or a simple home-grower or even by hand. It is also possible to prepare the layer using the method of spraying or dipping. The plate is dried and heated at about 100, pre-set, activate it. Place a solution of the sample in a volatile solvent with a pipette or syringe on the screen. When the dried stain leaves the plate perpendicularly in a suitable reservoir so that the lower edge of it is immersed in the moving phase, material separation is carried out u Affinity chromatography...
Thin Film Chromatography (TLC): Thin-layer chromatography is a type of , in which thin-film plates are used and the position of the components on the plate is determined. The particles on the layer should be large and compact and small. The quateary is often made up of , which is used to separate hydrophilic molecules such as carbon hydrates , amino acids , and Initially, chromatographic plates are required, ie, the gravity is distributed as a thin layer of uniform thickness on an inert rigid support. Glass sheets are commonly used, but there are other methods. Solid adsorbent in the form of fine powder with water, and sometimes with an escaping liquid , in the form of dough, and distributed by a commercial player or a simple home-grower or even by hand. It is also possible to prepare the layer using the method of spraying or dipping. The plate is dried and heated at about 100, pre-set, activate it. Place a solution of the sample in a volatile solvent with a pipette or syringe on the screen. When the dried stain leaves the plate perpendicularly in a suitable reservoir so that the lower edge of it is immersed in the moving phase, material separation is carried out u Affinity chromatography...ما را در سایت Affinity chromatography دنبال می کنید
برچسب : نویسنده : mfchemistry بازدید : 163
